
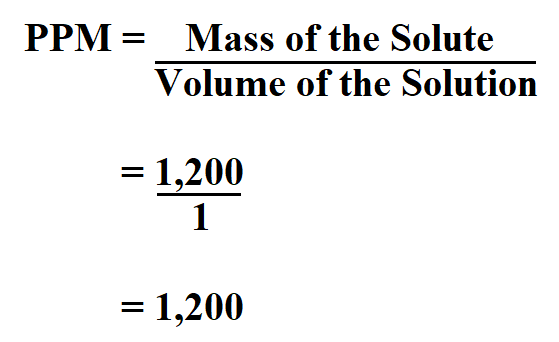
Parts-per notations may be expressed in terms of any unit of the same measure.
"2 ppb" would generally be interpreted as "two parts in a billion parts"). However, they generally take the literal "parts per" meaning of a comparative ratio (e.g. When parts-per notations, including the percent symbol (%), are used in regular prose (as opposed to mathematical expressions), they are still pure-number dimensionless quantities. In fractions like "2 nanometers per meter" (2 n m/ m = 2 nano = 2 × 10 −9 = 2 ppb = 2 × 0.000 000 001), so the quotients are pure-number coefficients with positive values less than or equal to 1. Parts-per notations are all dimensionless quantities: in mathematical expressions, the units of measurement always cancel. For instance, the accuracy of land-survey distance measurements when using a laser rangefinder might be 1 millimeter per kilometer of distance this could be expressed as " Accuracy = 1 ppm."

Parts-per notation is also employed to denote the change, stability, or uncertainty in measurements. For instance, a special metal alloy might expand 1.2 micrometers per meter of length for every degree Celsius and this would be expressed as " α = 1.2 ppm/☌". Similarly, parts-per notation is used also in physics and engineering to express the value of various proportional phenomena. Consequently, 1 ppm corresponds to 1 mg/L and 1 ppb corresponds to 1 μg/L. Therefore, it is common to equate 1 kilogram of water with 1 L of water. When working with aqueous solutions, it is common to assume that the density of water is 1.00 g/mL. The quantity "1 ppm" can be used for a mass fraction if a water-borne pollutant is present at one-millionth of a gram per gram of sample solution. Parts-per notation is often used describing dilute solutions in chemistry, for instance, the relative abundance of dissolved minerals or pollutants in water. 1.1 In nuclear magnetic resonance (NMR) spectroscopy.


 0 kommentar(er)
0 kommentar(er)
